Introduction: Bruton tyrosine kinase inhibitors (BTKi) are a mainstay of treatment for B-cell malignancies; however, their use can be limited by adverse events (AEs), many of which are potentially caused by off-target inhibition of other tyrosine kinases. The next-generation BTKi zanubrutinib was designed to maximize tolerability by minimizing off-target binding. Previous results from this ongoing phase 2 study (BGB-3111-215; NCT04116437) showed that zanubrutinib was well tolerated in pts intolerant of ibrutinib and/or acalabrutinib (Shadman et al. Lancet Haematol. 2023;10[1]:e35-e45). Here, we report updated results of the tolerability and efficacy of zanubrutinib in pts intolerant of acalabrutinib (cohort 2).
Methods: Eligible pts with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), Waldenström macroglobulinemia (WM), mantle cell lymphoma (MCL), or marginal zone lymphoma (MZL) who met protocol-defined criteria for intolerance of acalabrutinib received zanubrutinib 160 mg twice daily or 320 mg once daily. Pts whose disease progressed with prior BTKi therapy were excluded. Safety and efficacy, including recurrence of acalabrutinib intolerance events, were evaluated. Investigators assessed responses every 3 cycles based on standard response criteria for each indication using parameters at study entry as baseline.
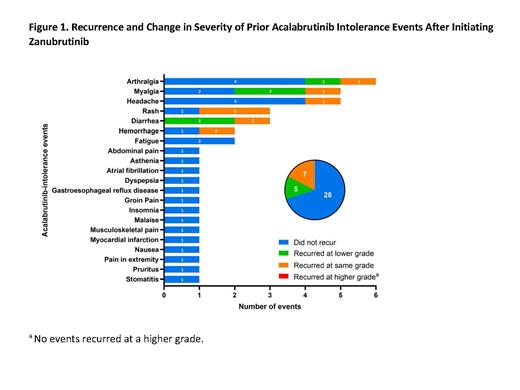
Results: As of May 15, 2023, 27 pts received zanubrutinib in cohort 2 (CLL/SLL, 19; WM, 4; MCL, 2; MZL, 2). Median age was 73 y (range, 51-87 y); median treatment duration was 11.4 mo (range, 0.5-32.2 mo), with median follow-up of 12.4 mo (range, 1.6-32.2 mo). Median number of prior therapies was 2 (range, 1-6); 13 pts (48%) received prior ibrutinib and acalabrutinib. Median cumulative exposure to acalabrutinib was 5.4 mo (range, 0.5-33.7 mo). Seven pts discontinued zanubrutinib (AE, n=2; physician decision, n=2; withdrawal, n=2; progressive disease, n=1) and 20 pts remained on treatment. A total of 40 acalabrutinib intolerance events were reported in 27 pts, most commonly arthralgia (n=6 events), headache (n=5), myalgia (n=5), diarrhea (n=3), and rash (n=3). Twenty-eight acalabrutinib intolerance events (70%) did not recur with zanubrutinib, corresponding to 17 pts (63%) not experiencing any recurrence of acalabrutinib intolerance events. Twelve events (30%) recurred (5 at a lower grade, 7 at the same grade, 0 at a higher grade; Figure 1) and 2 pts discontinued due to recurrence (myalgia and diarrhea; both recurred at the same grade); 1 pt discontinued due to investigator decision after the pt experienced a fall. Of 3 pts who experienced the same intolerance event with ibrutinib and acalabrutinib, 2 pts (1 experiencing atrial fibrillation and the other, pain in extremity) did not have a recurrence of these events with zanubrutinib, and 1 pt (experiencing diarrhea of grade 3 with ibrutinib and grade 2 with acalabrutinib) had a recurrence with zanubrutinib at a lower grade (grade 1). Although many pts entered the study with well-controlled disease, 24 of 25 efficacy-evaluable pts receiving zanubrutinib (96%) maintained or improved responses (at least stable disease) and 16 (64%) achieved a response better than stable disease compared with baseline.
Conclusions: With a median zanubrutinib exposure that was 6 months longer than the reported cumulative acalabrutinib exposure before discontinuation (11.4 vs 5.4 mo, respectively), 17 pts (63%) did not experience any recurrence of their prior acalabrutinib intolerance events, and disease was controlled in 24 of 25 efficacy-evaluable pts treated with zanubrutinib (96%). This suggests that pts who are intolerant of acalabrutinib can attain clinical benefit by switching to zanubrutinib. Enrollment and follow-up are ongoing.
Disclosures
Shadman:Mustang Bio: Consultancy, Research Funding; Eli Lilly: Consultancy; Genmab: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; MorphoSys/Incyte: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Fate Therapeutics: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; ADC therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding; Vincerx: Research Funding; Kite, a Gilead Company: Consultancy; MEI Pharma: Consultancy; Regeneron: Consultancy; TG Therapeutics: Research Funding. Flinn:Iksuda: Consultancy; Janssen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Juno: Consultancy, Research Funding; Karyopharm: Research Funding; Merck: Research Funding; MorphoSys: Research Funding; Seagen: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Verastem: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genmab: Consultancy; InnoCare Pharma: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Secura Bio: Consultancy; Servier Pharmaceuticals: Consultancy; TG Therapeutics: Consultancy, Research Funding; Vincerx Pharma: Consultancy, Research Funding; Xencor: Consultancy; 2seventy bio: Research Funding; Agios: Research Funding; ArQule: Research Funding; Biopath: Research Funding; Bristol Myers Squibb: Research Funding; CALIBR: Research Funding; CALGB: Research Funding; Celgene: Research Funding; City of Hope National Medical Center: Research Funding; Constellation Pharmaceuticals: Research Funding; Curis: Research Funding; CTI Biopharma: Research Funding; Epizyme: Research Funding; Fate Therapeutics: Research Funding; Forma Therapeutics: Research Funding; Forty Seven: Research Funding; IGM Biosciences: Research Funding; Incyte: Research Funding; Infinity Pharmaceuticals: Research Funding; Loxo: Research Funding; Marker Therapeutics: Research Funding; Millennium Pharmaceuticals: Research Funding; Nurix: Research Funding; Portola Pharmaceuticals: Research Funding; Rhizen Pharmaceuticals: Research Funding; Roche: Research Funding; Step Pharma: Research Funding; Tessa Therapeutics: Research Funding; Trillium Therapeutics: Research Funding; BeiGene: Consultancy; Seattle Genetics: Research Funding; Triphase Research & Development Corp.: Research Funding; Hutchison MediPharma: Consultancy; Great Point Partners: Consultancy; Gilead Sciences: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Century Therapeutics: Consultancy; BeiGene: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Acerta Pharma: Consultancy, Research Funding; Unum Therapeutics: Research Funding. Levy:Jazz: Consultancy, Honoraria, Speakers Bureau; Genmab: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau; Morphosys: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seagen: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Sellas: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squibb: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; AZ: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau. Farber:Atlantic Health System: Current Employment; BeiGene, Genentech, Genmab/Abbvie, Gilead/Kite, Incyte/MorphoSys, SeaGen: Speakers Bureau; Genmab/Abbvie, Oncology Society of New Jersey: Membership on an entity's Board of Directors or advisory committees. Cultrera:Acerta, BeiGene, Daiichi, Merck, TG Therapeutics, Sankyo Pharm: Research Funding. Crescenzo:BeiGene: Current Employment, Current holder of stock options in a privately-held company. Idoine:BeiGene: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Zhang:BeiGene: Current Employment, Current equity holder in publicly-traded company. By:BeiGene: Current Employment. Sharman:AbbVie, AstraZeneca, BMS, Beigene, Lilly, Genentech, Inc., Genmab: Consultancy; Merck, Novartis: Consultancy; Seattle Genetics: Research Funding; AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Inc., Lilly: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal